Bottom-Up
Synthetic Immunology
While each of these diseases could be controlled by our immune system, they all evade the immune response by similar mechanisms.
Table of Content
Table of Content
Goals
Define basic principles of immune control
Develop & experimentally validate candidates of a new class of engineered and synthetic therapeutics for clinical testing
Our approach
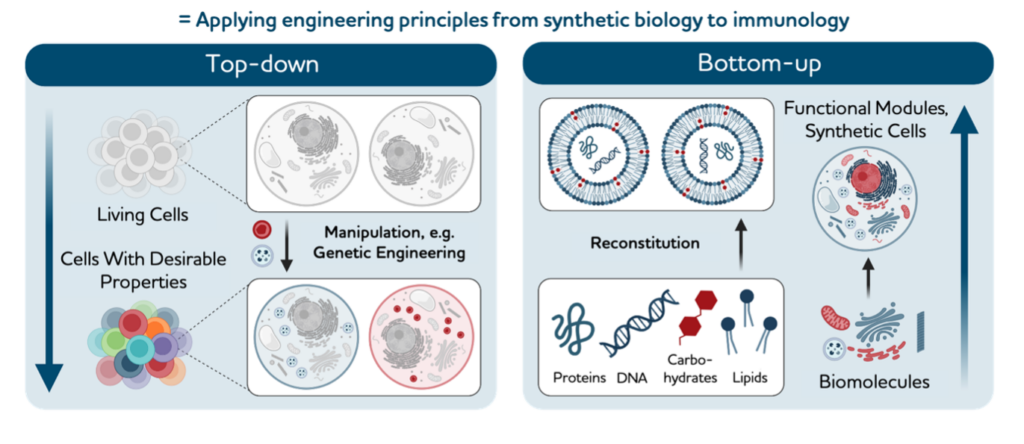
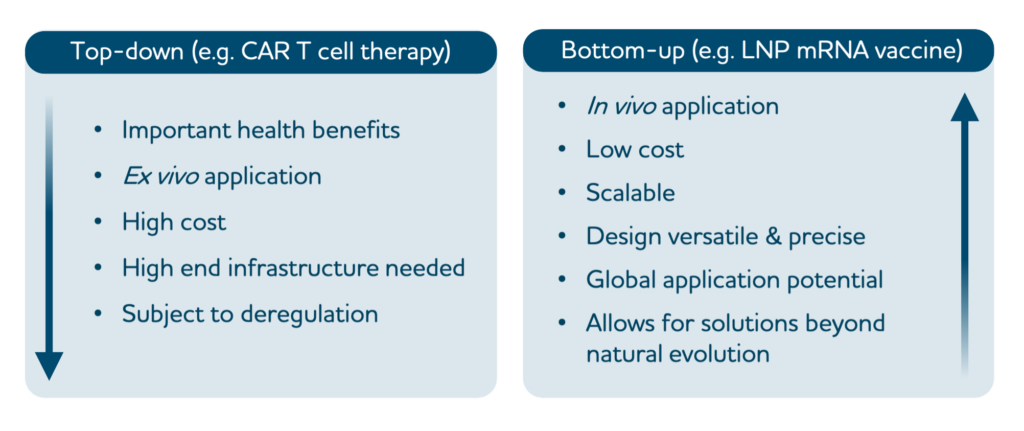
Synthetic biologists engineer cells by top-down genetic manipulation or bottom-up assembly from nanoscale building blocks. Recent successes in treating advanced tumours and diseases using genetically engineered immune cells highlight the power of the top-down synthetic immunology approach. However, genetic immune engineering is mostly limited to ex vivo applications and is subject to complex counter-regulation inherent to immune functions.
Bottom-up synthetic biology can harness the rich nanotechnology toolbox to engineer molecular and cellular systems from scratch and equip them with desired functions. These are beginning to be tailored to perform targeted immune functions and should hence allow intervention strategies by rational design. In this Perspective we conceptualize bottom-up synthetic immunology as a new frontier field that uses nanotechnology for crucial innovations in therapy and the prevention of infectious diseases and cancer.
Our complete strategy paper can be viewed here: Bottom-up synthetic immunology. Feel free to reach out to our office to receive a copy.
Top-down versus bottom-up synthetic immunology.
synthetic immunology modifies the natural immune system by altering existing immune cells or using components of the immune system to create desired therapeutic outcomes. In the example shown, CAR T cells from a patient are genetically engineered ex vivo to improve their functionality for therapeutic application in the patient.
synthetic immunology builds new immune functions from basic synthetic or natural building blocks to mimic or influence immune functions with superior control and specificity. In the example shown, mRNA vaccines are assembled from mRNA and lipid nanoparticles. Future bottom-up engineering approaches are expected to reach higher levels of complexity, including specific targeting, self-propulsion, slow release and ‘sense–compute–actuate’ modules.
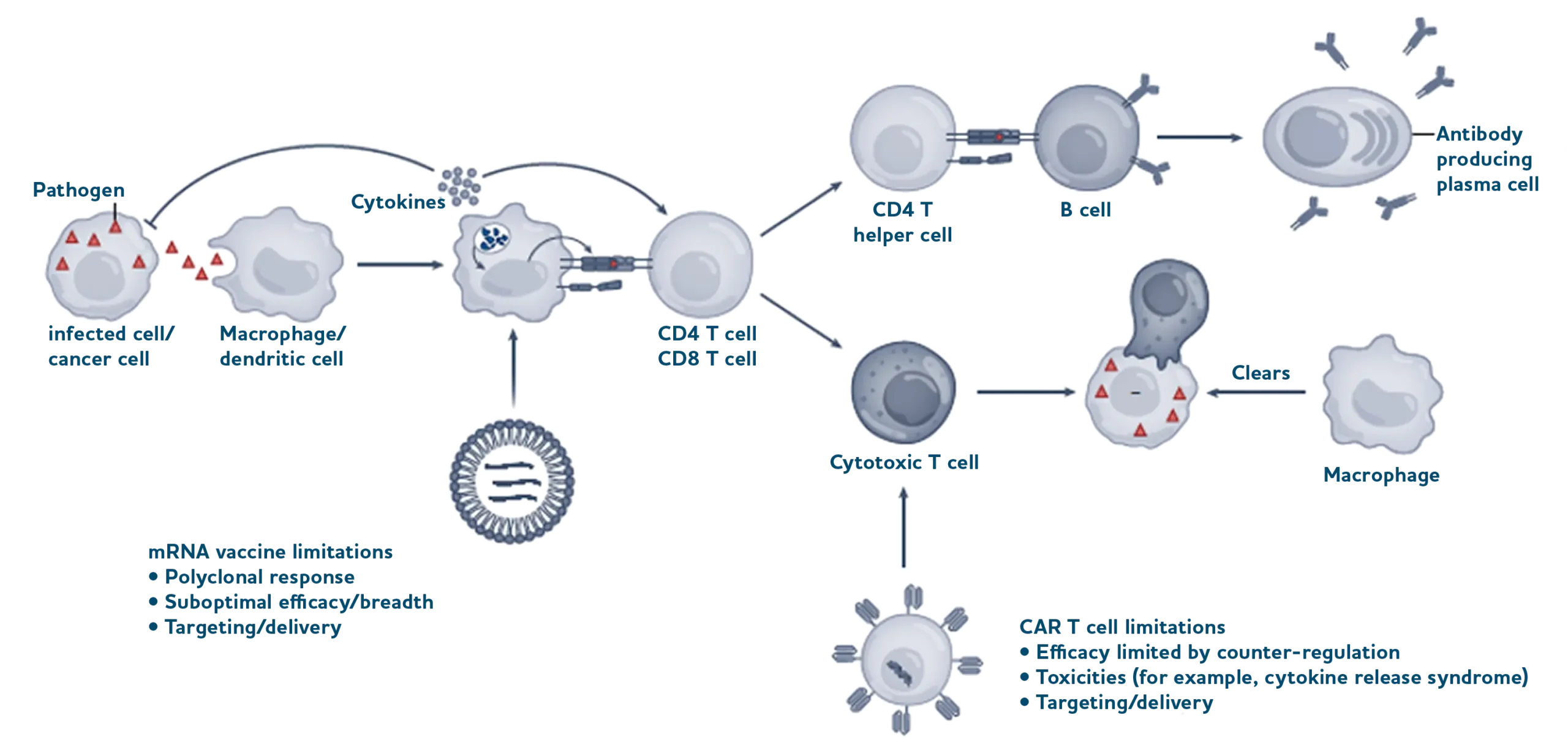
Schematic overview of innate and adaptive immune responses exemplified for pathogen infection.
Pathogens, as well as infected or cancer cells, are recognized by cells of the innate immune system (macrophage, dendritic cell), which represents a first line of defence through the immediate recognition of, and reaction to, danger signals (such as pathogen-associated molecular patterns; for example, viral RNA) by host cell pattern recognition receptors.
This triggers the expression of antiviral genes (for example, those that encode restriction factors) and cytokine responses (such as interferons), which can induce antiviral states in neighbouring cells and recruit more immune cells. Released cytokines also support adaptive immune reactions (adaptive immune cells in blue, such as IL-2 and IL-7 for T cells and IL-4 for B cells) that are tailored to the specific and dynamic antigenic properties of a pathogen and capable of providing long-term protective immunity. CD4 and CD8 T cells recognize specific antigens (parts of proteins that were digested by cells and presented by major histocompatibility complexes (MHCs) at the surface) for activation and differentiation.
The subsequent recognition of infected cells that expose this specific antigen can enable activated/cytotoxic CD8 T cells to kill infected cells, which are subsequently cleared by phagocytic cells such as macrophages (bottom). Activated CD4 T cells interact with antigen-specific B cells to help generate high-affinity antibody-producing plasma cells (top). Examples of limitations on how nanotechnology can help to trigger the expression of antigens for antibody production (mRNA vaccine) or exert engineered immune cell function (CAR T cell) are listed.